UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of Registrant as Specified in Its Charter)
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||||
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code:
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On September 15, 2022, Inari Medical, Inc. (the “Company”) made available in the investor relations section of its website a presentation, which includes an overview of the Company, and was presented at the Company’s Analyst and Investor Day on September 15, 2022. A copy of this presentation is attached as Exhibit 99.1 to this report, and the information set forth therein is incorporated herein by reference and constitutes a part of this report.
The information in this Item 7.01 (including Exhibit 99.1 hereto) shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Presentation of Inari Medical, Inc., dated September 2022 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| INARI MEDICAL, INC. | ||||||
| Date: September 20, 2022 | By: | /s/ Mitchell Hill | ||||
| Mitchell Hill Chief Financial Officer | ||||||

Exhibit 99.1 2022 Inari Medical Investor Day Sandy | Boynton Beach, FL

Financial Information Unless otherwise indicated, all financial and operational information included herein is as of June 30, 2022. Indications for Use and Publication References Indications for Use and relevant labeling information for all Inari products included in this presentation are included in the appendix. References to clinical studies and other publications cited in this presentation are located in the appendix. Forward Looking Statements This presentation (together with any other statements or information that we may make in connection therewith) may contain are forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including any estimates of revenue and total procedures, total addressable market, future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; our business model and strategic plans for our products, technologies and business, including our implementation thereof; competitive companies and technologies and our industry; our ability to grow and maintain our US sales force; our ability to develop new tools and new markets; the results of our clinical studies; our ability to commercialize, manage and grow our business by expanding our sales and marketing organization and increasing our sales to existing and new customers; third-party payor reimbursement and coverage decisions; commercial success and market acceptance of our products; our ability to accurately forecast customer demand for our products and manage our inventory; our ability to establish and maintain intellectual property protection for our products or avoid claims of infringement; FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally, including healthcare reform measures in the United States; the timing or likelihood of regulatory filings and approvals; our ability to hire and retain key personnel; our ability to obtain additional financing; and our expectations about market trends. Without limiting the foregoing, the words “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms and other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements are based on and reflect management’s current expectations, assumptions, estimates and projections that may or may not prove to be correct. These forward-looking statements are subject to a number of known and unknown risks, uncertainties, assumptions and other factors, many of which are beyond our control. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement. In light of these risks, uncertainties, and assumptions, the future events and trends discussed in this presentation may not occur and our actual results, results, levels of activity, performance or achievements could differ materially and adversely from those anticipated or implied by any forward-looking statements. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission (“SEC”), including our most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. These filings are available in the Investor Relations section of our website at https://ir.inarimedical.com/ or at www.sec.gov. The forward-looking statements in this presentation are made only as of the date hereof. Except to the extent required by law, we assume no obligation and do not intend to update any of these forward-looking statements after the date of this presentation or to conform these statements to actual results or revised expectations. All forward-looking statements are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements. This presentation is not an offer to sell securities of Inari Medical and it is not soliciting offers to buy securities of Inari Medical nor will there be any sales of securities of Inari Medical in any state or jurisdiction where the offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

Opening Remarks Bill Hoffman, CEO Amanda | Louisville , KY

Our dedication to changing lives drives every decision we make Amanda | Louisville , KY Jessica, 44 | Valparaiso, IN

We’ve made Patients first. Make no small plans. improving lives our Always. Ever. responsibility. And that drives our passion and success Take care of each other. Constantly.

Introduction To Inari Drew Hykes, COO Audry | Detroit, MI

Management Presentation Agenda Time Duration Session 9:00 – 9:20AM 20 min. Introduction 9:20 – 10:10 AM 50 min. Inari Five Growth Drivers 10:10 – 10:20 AM 10 min. Break 10:20 – 10:40 AM 20 min. Physician Panel 10:40 – 10:50 AM 10 min. Financials and Closing Remarks 10:50 – 11:30 AM 40 min. Q&A

Strong leadership team with breadth & depth Bill Hoffman Mitch Hill Drew Hykes Dr. Tom Tu Chief Executive Officer Chief Financial Officer Chief Operating Officer Chief Medical Officer Angela Ahmad Brian Strauss Eric Louw Janet Byk John Borrell Justin Crockett Kevin Strange Kit Cariquitan Eric Khairy General Counsel SVP Engineering SVP Marketing VP Manufacturing VP Finance & SVP Sales VP Inari Solutions VP Strategy & VP Quality Accounting Group Business Assurance & Development Reg. Affairs Norman Nie Paul Koehn Randy Hamlin Dr. Shon Chakrabarti Tara Dunn Dr. Venkat Tummala Dr. Victor Tapson Vitas Sipelis VP Information SVP Operations VP Advanced VP & General Manager SVP Clinical Affairs & VP Medical Affairs VP Medical VP International Technology Development Chronic Venous Market Development Affairs Diseases

Venous Thromboembolism (VTE) DVT Up to Develop Post-Thrombotic Syndrome (PTS) within 2 years of a proximal DVT 50% PE Leading cause of cardiovascular death #3

DVT PE 430,000 280,000 US Patients US Patients VTE is a large $3.0B $2.8B and highly TAM TAM underpenetrated Interventional Procedures Conservative Medical Management opportunity to serve patients $ in need 5.8B Total US VTE TAM Opportunity $ 15B+ Global VTE TAM Opportunity

Treatment of VTE evolving to definitive mechanical catheter intervention Myocardial Anti-Coagulation Definitive Catheter Lytics Based Only Therapy Infarction Anti-Coagulation Definitive Catheter Lytics Based Stroke Only Therapy Expected Path for Anti-Coagulation Definitive Catheter Lytics Based VTE Only Therapy (DVT & PE)

Highly differentiated, purpose-built solutions Simple, intuitive solutions Near complete thrombus removal Eliminate need for dangerous lytics Minimal blood loss Favorable hospital economics

Taking out all the clot matters

Our five growth drivers remain the roadmap Innovating Expanding Our Driving Deeper Building Clinical Expanding Into Purpose-Built 1 2 3 4 5 U.S. Sales Force VTE Penetration Evidence New Markets Solutions >$20B Total Global TAM 270+ <5% 250+ 5 + U.S. Sales Territories Penetration into U.S. VTE Peer Reviewed Publications Distinct Product Toolkits Incidence For 5 Distinct TAMs ~$10B US Prevalence Opportunity

Annual Revenue ($ in Millions) $300 Consistent, $250 133% premium $200 CAGR financial $150 $100 performance $50 $0 2019 2020 2021 $277M 91% 98% $330M 2021 Total Revenue 2021 Gross Margin YOY Growth (From FY20) Cash, Cash Equivalents, & Short-Term Investments (Q2 2022)

Our core competencies are scalable and allow us to treat more patients Identify major unmet R&D innovation engine to High-Touch, scalable Clinical infrastructure patient needs rapidly design purpose- commercial org & market generating data to change built devices development capabilities standard of care FOUNDATION OF OPERATIONAL AND MANUFACTURING EXCELLENCE

No small plans. And we’re just getting started EXPANDING US SALES FORCE BUILDING THE LARGEST INTERVENTIONAL SALES FORCE 1 DRIVING DEEPER PENETRATION STANDARDIZING PATIENT PATHWAYS 2 BUILDING CLINICAL EVIDENCE EXECUTING GUIDELINE-CHANGING CLINICAL TRIALS 3 INNOVATING NEW PRODUCTS DEVELOPING PURPOSE-BUILT SOLUTIONS 4 EXPANDING INTO NEW MARKETS LAUNCHING INTO NEW ADJACENCIES & GEOGRAPHIES 5

Growth Driver 1 Expanding Our US Sales Force John Borrell, SVP Sales

2020 (Q2) 2022 (YTD) Sales >3.5x ~75 >270 Significant Territories (US) growth in sales territories, Active increasing >2x ~650 ~1,400 Accounts (US) density of coverage Accounts per 0.6x ~9 ~5 Territory

PRODUCTIVITY REMAINS HIGH DESPITE GROWTH AND AGGRESSIVE TERRITORY SPLITS 350 100 90 300 80 Revenue Growth Focused on 250 70 60 growth but 3.6x 200 50 3.1x remaining 150 40 Number of Territories efficient 30 100 20 50 10 0 0

AVERAGE SALES REP PRODUCTIVITY OVER FIRST 18 MONTHS 20 2.5X Sales rep 18 16 productivity 14 12 ramps up 10 8 quickly after 6 start date 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Months After Start Cases Per Territory

Continuing growth to fully tackle VTE and address new disease states 2022 FUTURE STATE Potential to be the largest interventionally- >270 focused sales team US Sales Territories

COMMERCIAL / MARKET DEVELOPMENT TEAM 12 8 National Health Economics & Accounts Market Access (HEMA) Sales team efforts amplified by robust non-sales 7 7 Inari Solutions Medical Affairs commercial team Groups (ISG) (including doctors) 8 34 Medical Upstream/Downstream Education Group Marketing & Sales Ops.

Intentional fit-based hiring and promotion from within Single-tier sales team w/ ~90% case High-powered, high- presence touch commercial Mining information across all sources, informing every decision we make system designed to solve patient needs Solution-based toolkits, not widgets Deliberate territory splits & alignment of incentives

Our commercial system enables us to scale in new markets with significant unmet needs US VTE Global Leadership Inari High-Touch Commercial Global Markets Solving Patient System Unmet Needs New Large Unmet Needs

Growth Driver 2 Driving Deeper Penetration Eric Khairy, SVP Marketing

A significant responsibility Addressable US VTE ~710,000 patients per year

A significant responsibility 15-20% Receive any intervention Addressable US VTE ~710,000 patients per year

A significant responsibility 15-20% Receive any intervention <5% Treated with Inari products today Addressable US VTE ~710,000 patients per year

VTE lacks a systematic approach to identify, screen, and triage patients MYOCARDIAL STROKE VTE INFARCTION Consistent screening Consistent screening protocols protocols Algorithms for triage Algorithms for triage and treatment and treatment Tracked metrics Tracked metrics Interventionalist Interventionalist Interventionalist

VTE patients are inside the hospital, yet most never see a VTE expert Annual US VTE Incidence 710K US Hospital Beds ~740K Annual US Incidence per Bed ~1 500 Bed ~500 = Mid-sized hospital addressable VTE patients/year

We’re helping Excellent clinical outcomes hospitals build programs that Positive hospital economics connect VTE Systematic patient pathway patients to VTE (i.e., a “VTE program”) experts

VTE Excellence is a codified & scalable process to build VTE programs ENGAGE EMPOWER EXCEL ~120 Inari accounts ~20 Inari accounts ~1,250 Inari accounts Solidify consistent patient Find champions, Create patient pathway identification, triage, build the foundation and build awareness tracking

APPROX. ACCOUNT-LEVEL TAM PENETRATION BY STAGE 4x VTE Excellence activities are 22% beginning to drive deeper 16% account-level TAM penetration 5% Engage Empower Excel

What’s the future? Interventional TAM penetration in context Myocardial VTE Today Infarction ~90% 15-20% Interventional TAM Interventional TAM penetration* penetration *Intervention includes both CABG and angioplasty

Growth Driver 3 Building Clinical Evidence Tara Dunn, SVP Clinical Affairs Larry | Newport Beach, CA

Transforming patient care Do the right thing Generate data Develop Set the for patients with urgency the market bar high

Clinical by the numbers 2,000+ 250+ Patients studied Peer reviewed to date publications INCLUDING 2 RCTS 6 20+ Major prospective Active or completed Studies IIR engagements

Strong and versatile team driving the quality and pace of best-in-class evidence generation Clinical Clinical Biostats Scientific Insights Research Communication Real World Data Trial Clinical Data Dissemination Execution Analytics

A tsunami of clinical data PE Trials: FLASH interim RCT enrollment FLASH 800 FlowTriever Real-world all- late-breaking begins late-breaking System comers PE registry clinical trial clinical trial enrollment begins IDE enrollment begins High-risk PE Interim First high-risk st EU enrollment 1 mechanical study FlowTriever PE multicenter Publication begins thrombectomy enrollment FDA cleared series from device Published in begins FLASH indicated for PE JACC 2015 2016 2017 2018 2019 2020 2021 2022 st 1 mechanical RCT thrombectomy enrollment device 2 CLOUT begins CLOUT interim indicated for manuscripts late-breaking DVT clinical trial ClotTriever DVT Trials: FDA cleared ClotTriever Real-world DVT System CLOUT interim registry enrollment 2 CLOUT late-breaking begins late-breaking clinical trial clinical trials

Setting a high bar for VTE evidence PE STUDIES DVT STUDIES First Industry Sponsored Largest Prospective PE Largest Prospective First Inari RCT Largest Prospective DVT DVT RCT Device Study High-risk PE Device Study (FlowTriever v. CDT in PE) Thrombectomy Study (ClotTriever v. AC) ~1,000 Patients | 83 Sites 100+ Patients | 11 Sites 550+ Patients | 60 Sites 500 Patients | 47 Sites 300 Patients | 60 Sites th 800 & final US th Enrollment near Enrollment commenced in 500 & final Enrollment expected patient enrolled. complete both RCT and registry arms patient enrolled early 2023 EU enrollment underway ~2,500 patients across 5 studies Status

FLASH is the largest prospective registry in PE with exceptional results EXCELLENT IMMEDIATE LASTING PATIENT MORTALITY SAFETY PATIENT RELIEF BENEFITS FlowTriever® Mean Pulmonary System 10.2% Artery Pressure PERT Consortium -7.4 1.5% 0% mmHg Post-PE Syndrome Device related MAEs 1.3% All-Cause Mortality Pre-FT Post-FT (30d)

FLAME: High-risk PE guidelines are from an 8 patient study. FLAME is 100+ patients Changing Guidelines Designed per AHA recommendations Doing What's Right Up to 50% of high-risk patients die within 100+ Patients 30 days 11 Sites Developing the Market Why not treat intermediate-risk?

HIGHLIGHTS PEERLESS: Superiority Currently >60% of patients RCT of FlowTriever vs intervened on receive Catheter CDT in PE Directed Thrombolysis (CDT) Primary endpoint via win ratio: Intermediate-High Risk PE • All-Cause Mortality • Intracranial Hemorrhage • ISTH Major Bleeding Up to 150 Patients 550 Patients Randomized 1:1 • Clinical Deterioration/Bailout in Registry Arm • ICU Admission & ICU LOS Head-to-head definitive FlowTriever Only FlowTriever CDT (Contraindication cohort) treatment trial (US & EU) Patients Followed for 30 Days Enrollment ahead of schedule

EXCELLENT SAFETY CLOUT demonstrates 0% we can do better Vessel/Valve for DVT patients Damage ON-TABLE EFFECTIVENESS 86% 500 47 Effectiveness in Core Lab Patients Enrolled Sites Adjudicated Clot Removal LASTING PATIENT BENEFITS 2 out of 3 91% With Subacute and/or Chronic Clot Freedom from Moderate to Severe PTS at 6 months

HIGHLIGHTS DEFIANCE: Superiority RCT of ClotTriever vs First global industry- sponsored RCT for DVT Anticoagulation in DVT Primary endpoint via win ratio: • Treatment failure or escalation Moderate-Severe Iliofemoral DVT of therapy • Post-Thrombotic Syndrome severity at 6 months 300 Patients Randomized 1:1 Designed to transform standard of care Conservative Medical ClotTriever Management (AC) Enrollment expected to begin early 2023 Patients Followed for 6 Months

Exceptional program Enrollment began 2022 productivity and quality of patient outcomes Enrollment begins 2023 2021 Registry Expansion 1,000+ 2018 2018 2020 Global Definitive 2016 RCTs Global Studies US Real-Word Registries IDE

Growth Driver 4 Innovating New Products Dr. Tom Tu, CMO

Years of knowledge and commitment. Our mission to address unmet needs Protrieve Triever 24 Triever 20 Curve st 1 Gen FlowTriever st 1 Gen FlowTriever Triever 24 Flex ClotTriever XL Disks Artix FlowSaver 2015 2016 2017 2018 2019 2020 2021 2022 Intri24 InThrill FT 2 LBS 2 T20 Large Bore Syringe (LBS) ClotTriever nd 2 Gen BOLD rd FlowTriever 3 Gen FlowStasis Triever 16 ClotTriever Gen 4 Trievers ~70,000 patients treated | 38 world-wide patents

Our Innovation Engine continues to implement purpose-built solutions for unmet patient needs Rapid innovation “Engine Room” A Scalable, via in house machine shop, R&D infrastructure Repeatable System 3D-printing, bio-skills lab, laser cutting, etc. A Scalable, Repeatable 80 Full Time Engineers +9 UpstSy rea sm te M m arketing Engineering roles Mining information, defined around solutions, informing every project not products Market analysis focused on large and unmet needs

Large bore catheters produce large clot hauls

A comprehensive PE procedure solution years in the making Safely, Quickly Track Large Clot Hauls Address Challenging Minimal Blood Loss Throughthe Heart Without Lytics Clot or Anatomy Large Bore Fourth Generation Triever20 Curve® FlowSaver® Blood Aspiration Triever Catheters Catheter Return System Intri24® Large Bore Syringe and FlowTriever FlowStasis® Suture Sheath Whoosh Mechanism Catheters* Retention Device *The FlowTriever 2 catheter is not indicated for the treatment of PE

A purpose-built DVT treatment for the full range of clot chronicity Coring Element Collection Basket

A comprehensive DVT procedure solution years in the making Access Acute to Chronic Clot Removal Complex Procedures ClotTriever Gen 3 ClotTriever 13 Gen 3 ProTrieve™ Catheter Sheath Sheath ClotTriever 16 Gen 3 ClotTriever BOLD FlowTriever Sheaths Catheter Disk Catheters

TM Protrieve provides confidence during complex DVT and IVC procedures 20F sheath designed for right IJ access and optimal positioning within the IVC Wall apposing funnel designed to trap emboli Compatible with ClotTriever and FlowTriever platforms 34mm self-expanding nitinol mesh funnel allows full wall apposition in the IVC

Expanding beyond VTE to develop purpose-built solutions for new diseases VTE LEADERSHIP BROADER PERIPHERAL SOLUTIONS TM TM Artix Chronic Venous InThrill ClotTriever FlowTriever System Disease System System System Toolkit

CVD often progresses from DVT and includes scarred Chronic Venous vein walls & wall-adherent obstructions Disease (CVD) Chronic clot Post-Thrombotic occlusions If obstructions are left unaddressed, patients can PTS and venous leg develop painful, ulcers (VLU) debilitating ulcers

Conservative treatments for Chronic Venous Disease are inadequate and only address symptoms Compression Therapy Anticoagulation <50% 1 in 5 VLUs healed at 12 weeks VLUs never heal

ClotTriever BOLD was designed to extract the full range of clot chronicity Clot is often older than symptoms suggest ~30% greater radial force for improved wall apposition Improved thrombus engagement to treat the full range of acute to chronic DVT

We’re building a solution-based toolkit to address Chronic Venous Disease Remove Acute to Chronic Cross Chronic Treat Chronic Treat Venous In-stent Venous Thrombus Venous Occlusions Venous Occlusions Chronic Re-thrombosis ClotTriever BOLD Catheter Stent Cleaner Crossing Recanalization Device(s) Device Tool Launched 2022 In development In development In development

Arterial Thromboembolism Acute limb ischemia (ALI) Acute embolization event – extensive damage can happen if Acute visceral ischemia not treated quickly Chronic limb ischemia (CLI)

Often requires open surgical procedures Current treatments for Distal embolization and vessel trauma arterial thrombo- High rates of lytic use embolism have Significant blood loss significant drawbacks Need for a better, purpose-built solution

The Artix System: purpose-built toolkit for peripheral artery thromboembolism Artix MT Combines both aspiration and mechanical Device thrombectomy Sheath has 4X flow rate vs. existing arterial catheters Artix BG Balloon Guiding Sheath Proximal flow arrest to prevent distal embolization
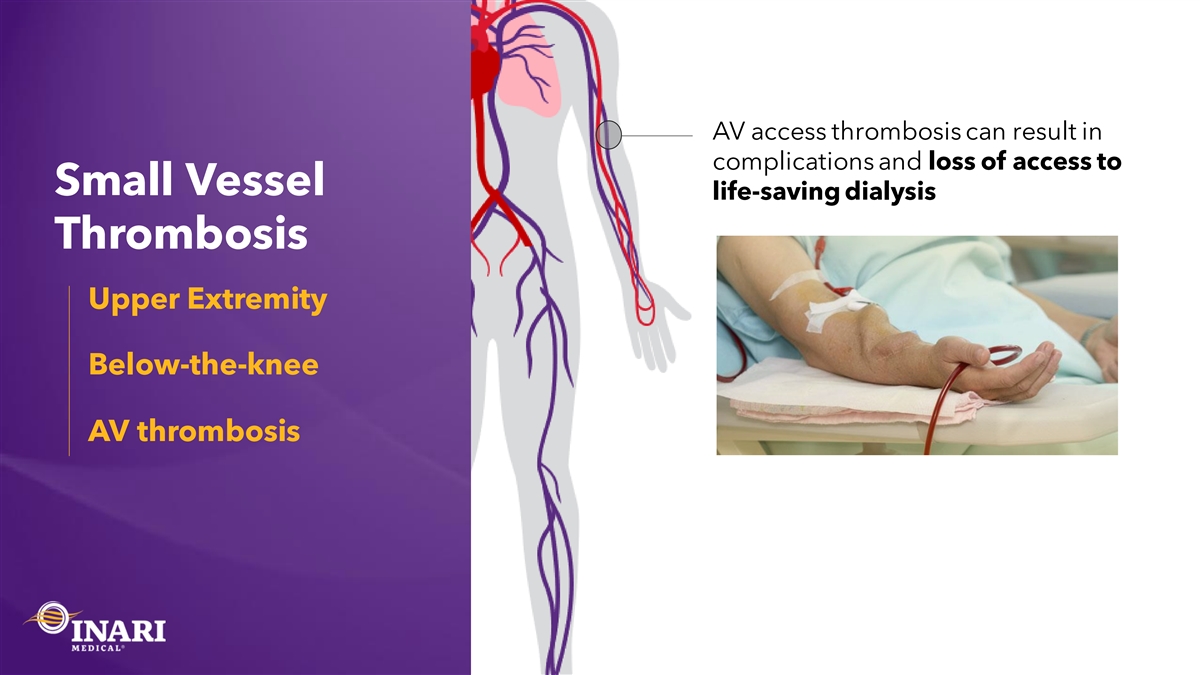
AV access thrombosis can result in complications and loss of access to Small Vessel life-saving dialysis Thrombosis Upper Extremity Below-the-knee AV thrombosis

AV “declotting” sends clot to the lungs, Limitations in exacerbating pulmonary hypertension current treatments High recurrence rates for small vessel Ineffective for chronic clot thrombosis Ineffective for large clot burdens Need for a better, purpose-built solution

The InThrill System: a solution for smaller vessels Self- expandable, laser-cut Effectively extracts clots element Addresses acute to chronic thrombus InThrill Catheter Tailor made for 4-10mm vessels InThrill Sheath Note: the InThrill device is indicated for use in the peripheral vasculature

We’re just getting started! ~ ~ 25+ 5K 70K Projects Across Cumulative Cumulative Current and Patients Patients New TAMs Treated Treated Protrieve T20 Curve Intri24 ClotTriever Gen 1-3 FlowTriever Gen 1-3 FlowStasis FlowSaver InThrill Future Purpose-Built Devices Coming FlowTriever Disks Triever16, 20 & 24 FlowTriever 2 CT Bold Artix <2020 2020 - 2022 2022 – 2024+ ~70K cumulative patients treated since inception of company

Growth Driver 5 Expanding into New Markets Drew Hykes, COO

Historically, we have treated patients in two TAMs with two toolkits 2 PE + DVT TAMs

+3 TAMs Currently expanding into three Chronic Venous Disease new TAMs, Arterial Thromboembolism Small Vessel Thrombosis continuing to address large unmet needs 2 PE + DVT TAMs

+3 TAMs More purpose-built We have no solutions to come small plans. More purpose- Chronic Venous Disease built solutions Arterial Thromboembolism to come in Small Vessel Thrombosis incremental TAMs 2 PE + DVT TAMs

Protrieve is a purpose-built solution for complex IVC cases LMR launched in August Enables complex IVC cases New tool for interventionalists treating DVT and PE $4K ASP affords incremental revenue

CHRONIC ACUTE CHRONIC VENOUS THROMBUS THROMBUS DISEASE Chronic Venous Disease prevalence opportunity larger than core TAM INCIDENCE PREVALENCE ~ ~ ~ ~ 100K $1B 1M $10B Patients TAM Opportunity Patients TAM Opportunity

Chronic Venous Disease: addressing the underlying cause via purpose-built devices ~3,000 CT-Bold cases Recanalization completed “Bold is amazing Device(s) for treating more-chronic In development ClotTriever BOLD Evaluating go-to-market options Launched Mar 2022* patients… but I CANNOT WAIT for what is coming with the Common interventional call point rest of this Crossing Stent Cleaner dedicated Device Device toolkit!” In development In development Premium ASP of ~$10K – Dr. Nicolas Mouawad *To treat acute to chronic clot

Arterial • Acute event – extensive damage can happen if Thromboembolism not treated quickly large TAM with • Includes Acute Limb Ischemia, Acute Visceral significant unmet Ischemia, and CLI procedures where distal needs embolization occurs ~ ~ 80K $600M US Patient Incidence US TAM Opportunity

Artix: purpose-built toolkit addressing unmet needs in arterial thromboembolism Artix MT LMR in April 2022; Balloon Sheath LMR in July 2022 No wonder “ lytics didn’t ` Significant site-of-service and physician work on that. overlap – Dr. Jerry Chung Targeting an ASP of ~$7.5K Additional tools in development

Small Vessel AV access Thrombosis: thrombosis can result in No purpose-built complications and loss of solutions exist for access to life- this large patient saving dialysis population ~ ~ ~ 150-200K 80K $1B US AVF thrombotic Addressable US BTK + UE Total US Market events / year thrombotic events / year Opportunity

InThrill: a purpose-built, novel solution designed to treat small vessels LMR launched in August Targeting in-hospital procedures; significant treating physician overlap with DVT and PE We’v“ e been waiting a long Targeting an ASP of ~$4K time for a mechanical option like this that works. Additional tools in development – Dr. John Ross

~$ Pulmonary Embolism 2.8B Deep Vein ~$ Small Vessel 3.0B ~$ Thrombosis 1.0B Thrombosis Arterial Large US total ~$ 0.6B Thromboembolism addressable market totaling ~$ Chronic Venous 1.0B Disease ~$8B across 5 Incidence disease states Does not include ~$10B incremental prevalence opportunity

> $20B Substantial Global Incidence TAM global across 5 Disease States opportunity exists across VTE and three new disease ~ states $10B CVD Prevalence Opportunity (US only)

Laying the foundation to treat patients globally Cases Completed Approval Received Approvals In-progress CASES COMPLETED IN 20+ COUNTRIES ACROSS THE GLOBE

No small plans. And we’re just getting started EXPANDING US SALES FORCE BUILDING THE LARGEST INTERVENTIONAL SALES FORCE 1 DRIVING DEEPER PENETRATION STANDARDIZING PATIENT PATHWAYS 2 BUILDING CLINICAL EVIDENCE EXECUTING GUIDELINE-CHANGING CLINICAL TRIALS 3 INNOVATING NEW PRODUCTS DEVELOPING PURPOSE-BUILT SOLUTIONS 4 EXPANDING INTO NEW MARKETS LAUNCHING INTO NEW ADJACENCIES & GEOGRAPHIES 5

10 Minute Break

Physician Panel Dr. Tom Tu, CMO

Inari Investor Day Physician Panel Discussion Christopher M. Huff, MD Interventional Cardiology = OhioHealth Riverside Methodist Hospital Thomas Tu, MD Chief Medical Officer = Inari Medical Moderator Steven Abramowitz, MD Vascular Surgery = MedStar Washington Hospital Center

Financials Mitch Hill, CFO
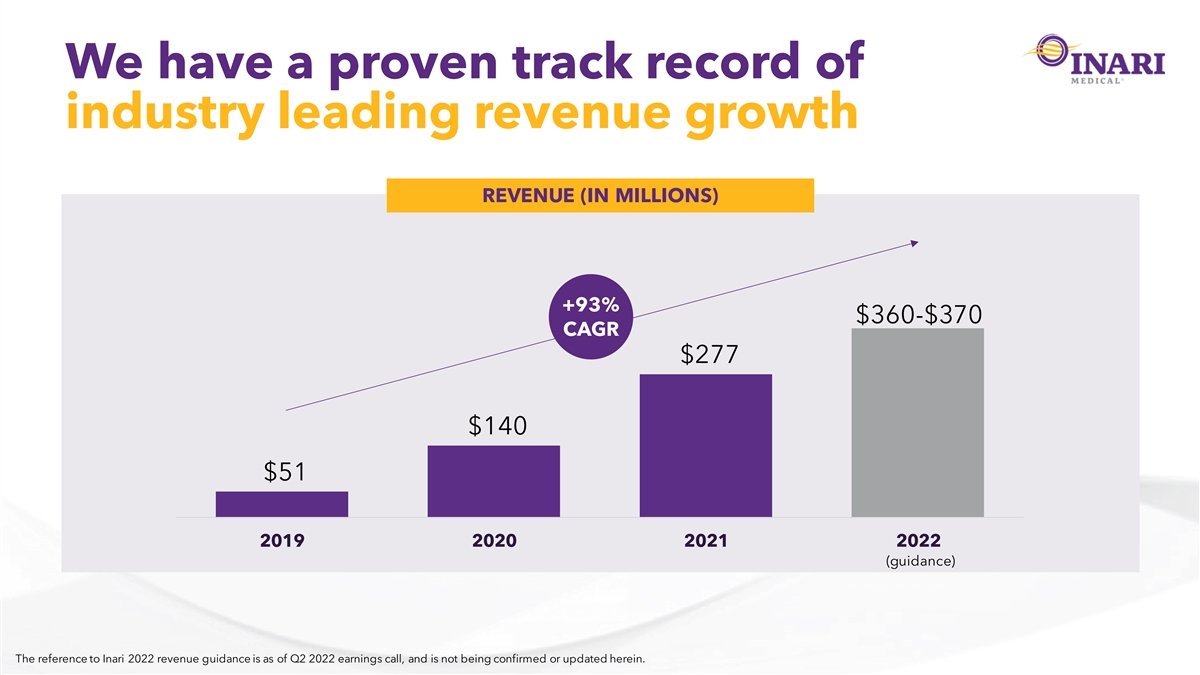
We have a proven track record of industry leading revenue growth REVENUE (IN MILLIONS) +93% $360-$370 CAGR $277 $140 $51 2019 2020 2021 2022 (guidance) The reference to Inari 2022 revenue guidance is as of Q2 2022 earnings call, and is not being confirmed or updated herein.

MedTech comps to approximate future VTE market size VTE PENETRATION TODAY INTERVENTIONAL PENETRATION PROXIES 15-20% Interventional Penetration Today ~30% ~90% $5.8B Myocardial Acute Ischemic TAM Infarction Stroke

Substantial revenue opportunity exists for the leader in $5.8B VTE TAM INARI REVENUE POTENTIAL 60% $1B+ $2B+ $1B+ 30% Inari 20% Today 30% 60% VTE Interventional Market Share (Procedures) VTE Interventional Penetration

Premium growth combined with exceptional margin profile GROSS MARGIN 91.1% 90.6% 88.4% 88.7% 2019 2020 2021 2022(1H)

Well-positioned for sustained operating profitability 2022 2024+ Significant investment Sustained operating in our growth drivers profitability by 1H 2024 • Large, attractive market • Commercial team • ~85% target gross margin • Clinical research • Commercial productivity ramp • Product development pipeline • Disciplined investment approach • International

All the components of a premium financial profile Market leader in $5.8B underpenetrated US VTE market; expanding into global ~$20B+ TAM Exceptional gross margin profile Disciplined investments driving growth, operating leverage and consistent profitability Strong balance sheet and ~$330M cash position, allowing financial flexibility Reference to cash position includes cash, cash equivalents, & short-term investments as of Q2 2022.

Q&A

Closing Remarks

Appendix

Citations Slide #: Source(s): 12 • Kahn, Susan R. Hematology Am Soc Hematol Educ Program. 2016 Dec 2; 2016(1): 413–418 12 • Pulmonary Embolism in 2017: Increasing Options for Increasing Incidence, National Center for Biotechnology Information, May 2017 • Hospital claims data 13, 17, 83 • Global Burden of Disease Data per the Institute for Health Metrics and Evaluation (IHME) 31, 32, 91, 92 • Hospital claims data and internal analysis 34 • Hospital claims data and internal analysis 38 • Hospital claims data 46 • Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113(4):577-82 47 • Hospital claims data and internal analysis • Fife, C. E., Publicly Reported Wound Healing Rates: The Fantasy and the Reality. Advances in wound care 2018 61 • Fife, C.E., From the Editor: The Need for Real Venous Ulcer Data. Today’s wound clinic., 2018 • Donadini, Marco, et. al., “Prognostic Significance of Residual Venous Obstruction in Patients with Treated Unprovoked Deep Vein Thrombosis.” Thrombosis and Haemostasis, vol. 111, no. 01, 2014, pp. 172–179 76 • Dronkers, C.E.A. et. al., Deep vein thrombosis : diagnostic and prognostic challenges Thromb Haemost. 2018 Aug; 118(8):1428-1438 • Hospital claims data and internal analysis • 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2021 • Stolic R: Most Important Chronic Complications of Arteriovenous Fistulas for Hemodialysis. Med Princ Pract 2013;22:220-228 78 • Quencer KB, Oklu R. Hemodialysis access thrombosis. Cardiovasc Diagn Ther 2017;7(Suppl 3):S299-S308 • Internal analysis • Fleck D, Albadawi H, Wallace A, Knuttinen G, Naidu S, Oklu R. Below-knee deep vein thrombosis (DVT): diagnostic and treatment patterns. Cardiovasc Diagn Ther. 2017 Dec;7(Suppl 3):S134-S139 • Elna M. Masuda, Robert L. Kistner, The Case for Managing Calf Vein Thrombi With Duplex Surveillance and Selective Anticoagulation, Disease-a-Month, 78 Volume 56, Issue 10, 2010, Pages 601-613, ISSN 0011-5029 • Franco, L, Giustozzi, M, Agnelli, G, Becattini, C. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta-analysis. J Thromb Haemost 2017; 15: 1142– 54 • Hospital claims data and internal analysis • Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012 Jun 7;366(23):2198-206 • Howard et. al., Population-Based Study of Incidence, Risk Factors, Outcome, and Prognosis of Ischemic Peripheral Arterial Events. Circulation Vol 132, Issue 19:1805–1815 80 • Conte MS, et. al., GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019 Jun;69(6S):3S-125S.e40 • Agarwal S, et al. Burden of Readmissions Among Patients With Critical Limb Ischemia. J Am Coll Cardiol. 2017 Apr, 69 (15) 1897–1908 • Internal analysis

Device Indications For Use The FlowTriever System® is indicated for (1) the non-surgical removal of emboli and thrombi from blood vessels (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The FlowTriever System is intended for use in the peripheral vasculature and for the treatment of pulmonary embolism. Triever Catheters are intended for use in peripheral vasculature and for the treatment of pulmonary embolism. The Triever Catheters are also intended for use in treating clot in transit in the right atrium. The FlowTriever2® Catheter is indicated for the non-surgical removal of emboli and thrombi from peripheral blood vessels. Injection, infusion, and/or aspiration of contrast media and other fluids into or from a blood vessel. The FlowTriever2 Catheter is intended for use in the peripheral vasculature. The FlowSaver® Blood Return System is used with Triever Catheters for autologous blood transfusion. The Intri24 introducer sheath is indicated to provide a conduit for the insertion of endovascular devices into the vasculature while minimizing blood loss associated with such insertions.

Device Indications For Use (cont.) TM The ClotTriever thrombectomy system consists of the ClotTriever catheter and ClotTrieversheath. The ClotTriever Thrombectomy System is indicated for: (1) the non-surgical removal of thrombi and emboli from blood vessels. (2) Injection, infusion, and/or aspiration of contrast media and other fluids into or from a blood vessel. The ClotTriever Thrombectomy System is intended for use in the peripheral vasculature including deep vein thrombosis (DVT). The FlowStasis® Suture Retention Device is indicated for temporary suture retention following a percutaneous venous procedure. The Artix MT thrombectomy device is indicated for: (1) The non-surgical removal of emboli and thrombi from blood vessels. (2) Injection, infusion, and/or aspiration of contrast media and other fluids into or from a blood vessel. The Artix MT thrombectomy device is intended for use in the peripheral vasculature. The InThrill Thrombectomy System consists of the InThrill Thrombectomy Catheter and InThrill Sheath. The InThrill Thrombectomy System is indicated for: (1)The non-surgical removal of thrombi and emboli from blood vessels. (2) Injection, infusion, and/or aspiration of contrast media and other fluids into or from a blood vessel. The InThrill Thrombectomy System is intended for use in the peripheral vasculature. The InThrill Thrombectomy System is not intended for use in deep vein thrombosis (DVT) treatment.

Device Indications For Use (cont.) Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. Refer to Instructions for Use for complete indications for use, contraindications, warnings, and precautions. All trademarks are property of their respective owners.
